Is the future of patient cancer treatment in trouble?
Dr.M.Raszek
Cancer survival report card
Merogenomics had an opportunity to do some research work for one of the Canadian companies that specializes in cancer genomics. While searching for information related to the novel uses of targeted therapies guided by the sequencing of cancer biopsies, many facts emerged that, instead of making the future of oncology more optimistic, potentially paint a more problematic picture. Not that we don’t have much to rejoice about already when it comes to battling cancer, from improved survival rates to many new blockbuster personalized medicines emerging. But behind this initial luster of exciting news also comes some troubled news that might jeopardize access of many future patients to the best treatments and best outcomes.
Yes, there is much to rejoice, as trends suggest that the battle against cancer is slowly being won. Perhaps it’s a tortoise race, but the metrics show improvements. While still more than 600 thousand people are expected to perish from cancer in the US in 2018 alone (the second leading cause of death for men and women), the latest 2018 cancer statistics data show that the death rate has been declining by about 1.5% annually in both men and women over the past decade. Maybe that does not sound like much, but it translates to a 26% reduction in cancer death rates since 1991! And for some individual cancers, these numbers are astonishing. “Specifically, the death rate dropped 39% from 1989 to 2015 for female breast cancer, 52% from 1993 to 2015 for prostate cancer, and 52% from 1970 to 2015 for colorectal cancer. Lung cancer death rates declined 45% from 1990 to 2015 among males and 19% from 2002 to 2015 among females due to reduced tobacco use”. In the meantime, the incidence rate remains the same in women, while dropping in men at an annual rate of 2% in that time span.
The picture might not seem as rosy, but the Canadian cancer mortality and incidence rate trends appear very similar to our neighbours down south. From 1992 to 2012, the death rates decreased by 29% in both men and women for colorectal cancer, 36% for female breast cancer, 41% for prostate cancer, and 35% for lung cancer in males. Unfortunately, the mortality from lung cancer in women increased by nearly 19% in that time span. Also in that time span, for the listed cancers the incidence rate was dropping in both genders at nearly a 2% annual rate.
Back to the US, since the mid-70s, overall survival has improved for all cancers except uterine system cancers. In fact, three cancers (pancreas, skin, and breast in women), now boast 5-year survival rates at over 90%. The worst are still the ones you expect to be with pancreas, liver, and lung cancers at less than 20% of 5-year survival. Of course these numbers change depending on the stage of cancer, and unfortunately lung and pancreatic cancers are typically discovered at later stages when it is pretty much game over, as the cancer has done its worst damage. However, the overall survival rate for all cancers is 68% in white-skinned individuals and 61% in black-skinned individuals (although some cancers exhibit astonishing differences between ethnicities).
One contributing factor, investigated in many clinical trials past and present, is the use of novel targeted therapies. The research definitely seems promising, and we shall leave that data analysis for another post. But many novel targeted therapies have come out on the market in recent years, and one way to take advantage of them requires testing the cancer biopsy for its mutated DNA.
Here is one potential problem facing our future cancer patients. While very promising, and rapidly being adopted by cancer centers around the world, the molecular analysis of patient tumours is definitely not the norm yet, and one of the contributing factors is its cost. Cancer is already prohibitively expensive to treat, so what additional costs are we talking about?
One of the analysis in the clinical cost of using the genome sequencing application towards treating patients comes from our very own British Columbia Cancer Agency that is overseeing the Personalized OncoGenomics (POG) program. The POG program was launched in 2012 to investigate DNA and RNA sequencing “to guide real‐time treatment planning for patients with treatment‐resistant cancers“. And one of the goals of the program was to estimate the average genome analysis cancer patient cost.
The cost assessment was made based on 301 patients, and we might as well show what cancer types were found in these patients, as it reflects the type of cancers that are treated on average at the BCCA.
So what does it cost our taxpayers to help determine the molecular profile of cancer patients? On average, whole genome analysis cost $34,886 Canadian dollars per patient in 2015, with DNA and RNA sequencing responsible for the bulk of that cost, with a mean cost of $19,400 per patient.
So that’s pretty expensive, and definitely not within the realm of availability to every patient who will be diagnosed with cancer. Forget about it for now. It is not going to get much better any time soon, as modelling of future costs suggests that mean costs associated with biopsy sequencing will not reach $5000 per patient within the next 10 years. If you assume a 1% monthly decrease in costs from now, on top of the already observed pattern, then we are looking at the forecasted mean costs reaching $5000 per patient in 2023, $3000 in 2024, and $1000 in 2025. Hmmmmm, $1000 for cancer genome sequencing and analysis is probably not likely, considering that the current biopsy processing costs are half of that price tag alone.
This means that genomic molecular profiling for cancer patients will be largely inaccessible for the vast majority of patients for many years to come.
The picture does look bit rosier for the DNA and RNA sequencing costs alone though. Under the basic pattern model, these costs will reach $5000 per patient by end of 2019, $3000 in 2020, and $1000 in 2021. Under the second scenario, incorporating a 1% monthly decrease in costs, sequencings costs reach $5000 per patient in 2018, $3000 in 2019, and $1000 at the beginning of 2021. And that seems pretty realistic to me, closely reflecting current market prices.
Swelling cancer patient numbers with not enough doctors
There is another problem associated with an increased number of cancer patients needing to see their doctors. Those fewer doctors that are already going to be overburdened will have another difficulty on their hands. And we don’t mean not enough chairs in their clinic lobby! The increasing aging population of cancer survivors, and the new patients that will be demanding more visits from their oncologists, will also be facing additional health problems. Together these comorbidities will only make cancer treatment that much more difficult and complex, requiring even greater expertise and refined knowledge from already overwhelmed physicians.
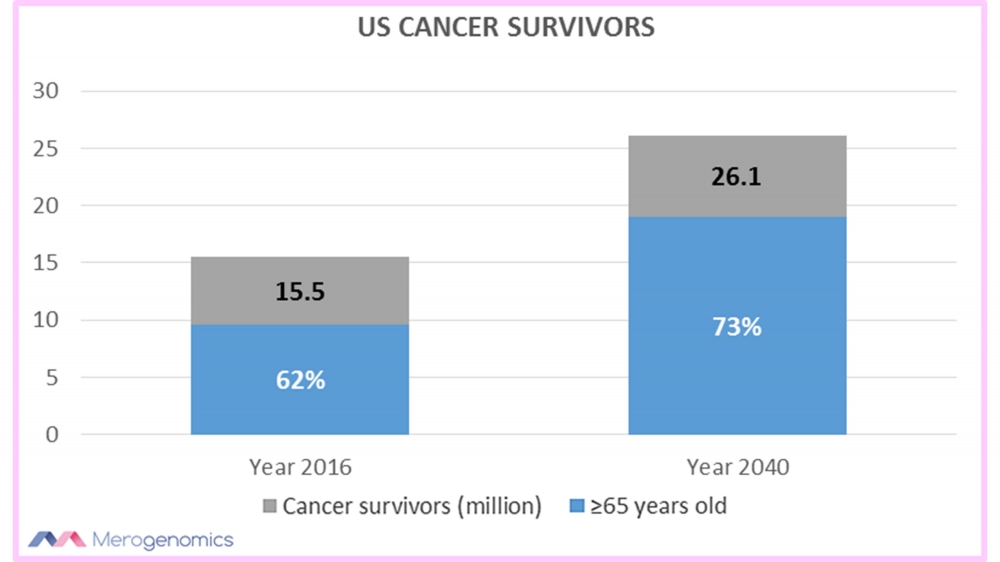
One research into the topic of aging cancer survivors suggested that in 2016, there were around 15.5 million cancer survivors in the United States alone, with 62% being 65 years or older. And these numbers are going to swell dramatically with the projected number of cancer survivors at 26 million by 2040, with 73% of them being 65 years and older.
This means a lot more work, a lot more treatments, and lot more costs, so we better be ready.
The real emerging problem is that while we are facing an ever increasing number of cancer patients to deal with, the corresponding number of oncologists to meet these needs are not increasing at the same rate. One scientific study that investigated the problem concluded that by 2025 the need for oncologist services will increase by 40% in the US (accounting for more than 83 million visits with cancer specialists), but the number of oncologists to meet these demands will increase only by 25%. Just to break it down further for you, 32% of these visits will be for chemotherapy and 85% for radiation, but we sincerely hope that targeted therapies will take a good chunk out of these numbers in the coming years.
On top of that, one even older study to tackle that same issue of demand versus supply of oncologists, that was raising alarms already in 2007, also stated that half of the American practicing oncologists could be retiring by 2020.
Both of these studies also showed that female oncologists tend to have fewer patient visits than male counterparts, in part due to a more personalized approach to each patient. While patients who already complain that their oncologists do not pay enough attention to their health problems secondary to cancer obviously love the extra attention provided by female oncologists, the welcomed increase of female doctors will inadvertently also contribute to decreased access to oncology specialists.
So we are faced with a very serious situation of a shortage of oncologists in the near future, which very likely will negatively impact cancer patient quality care, and exhaust our oncologists.
Health of our oncologists
These increasing burdens in cancer care will likely have undesirable outcomes for the doctors too. Symptoms of stressed situations are already being documented. Oncologists already face the increased psychological burdens of dealing with more deaths of their patients than seen in other medical professions.
One Canadian study into this issue showed that more than half of the surveyed oncologists have to deal with more than 3 patient deaths per month. More than 80% of them reported that patient age, long-term management, and unexpected disease outcomes are all contributing factors to patient death being more emotionally difficult. These are all the factors we already discussed which we are all likely to only see more of.
The oncologists rank lowest for work-life balance satisfaction among all of the medical specialties. In a perhaps ironic twist, female oncologists and those devoting greater time to patient care were found less likely to be satisfied with work-life balance. In turn, diminished satisfaction with work-life balance, along with professional burnout, were found to be the strongest incentives towards oncologists desiring to leave their position or retire early. Of course it is a self-feeding loop, where poor work-life balance and a strenuous work load lead to an oncologist’s burnout and compassion fatigue. So if your doctor seems emotionally distant, or like they don’t care, take it easy on them, they are simply coping with intense emotional duress. Perhaps most tragically of all, doctors exhibit higher suicide rates than those of the general population or other academics, especially among women.
These are all very alarming trends that should not be ignored, and demand public attention. Both for the benefit of our patients, which when it comes to cancer appear to potentially be anyone, as well as for our treating doctors. Considering the enormous impact of societal well-being that we place in the hands of our doctors, perhaps their well-being should especially command a high level of attention, and ensure that their mental health is addressed.
While many solutions have been proposed in some of the above quoted references, we will provide one more, and that is to increase public awareness and access to targeted therapies. There are many potential advantages to consider here. One could be the simple fact of improved outcomes among patients, along with the reduced need for treatment trial-and-error. The selection of targeted therapies based on the cancer molecular profile is still not a widely used option among oncologists, and typically has to be considered as an out-of-pocket expense for the patient. That, however, can potentially also alleviate the workload burden of oncologists, as some of the research and treatment selection is off-loaded from doctors using scarce public resources. Given the opportunity to understand the differences between the currently approved personalized targeted therapies as compared to standard approaches, we would not be surprised if a large subset of the cancer patient population would be selecting such out-of-pocket expenses. This is in essence the purpose behind Merogenomics, to help to bridge the gap between what is currently available in public health care and the advances in DNA sequencing technologies already realized, especially in cancer DNA tests.
So while the future of cancer care might look worrisome, hope should always remain.
This article has been produced by Merogenomics Inc. and edited by Kerri Bryant. Reproduction and reuse of any portion of this content requires Merogenomics Inc. permission and source acknowledgment. It is your responsibility to obtain additional permissions from the third party owners that might be cited by Merogenomics Inc. Merogenomics Inc. disclaims any responsibility for any use you make of content owned by third parties without their permission.
Products and Services Promoted by Merogenomics Inc.
Select target group for DNA testing
Healthy screening |
Undiagnosed diseases |
Cancer |
Prenatal |
Or select popular DNA test
 |
 |
 |
 |
Pharmaco-genetic gene panel |
Non-invasive prenatal screening |
Cancer predisposition gene panel |
Full genome |